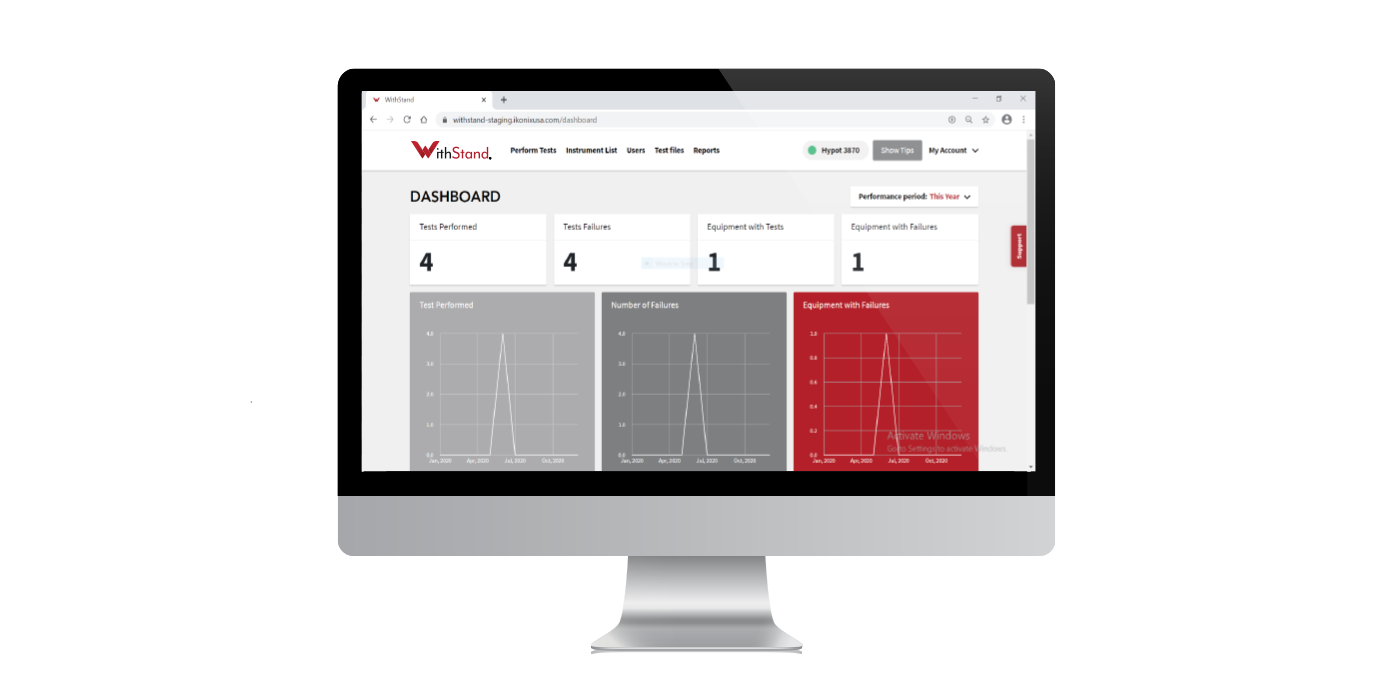
Compliance with medical testing standards is a challenge for all medical device manufacturers. We can help you find the right solution to ensure you are performing compliance and production testing as efficiently and effectively as possible.
Complete Solution
To meet the requirements of IEC/UL 60601-1, 3rd edition, we recommend performing functional and power consumption tests on your medical devices. For a complete test solution we recommend our 8500 Series, Associated Research OMNIA® II (8206 or 8207 depending on your testing requirements), and SC6540 paired with our WithStand® software.
Introductory Solution
Medical Device manufacturers should perform functional test as a 100% production test. As an introductory solution we recommend our 8500 Series paired with our SCI 446 and PowerTRAC™ software. With PowerTRAC you are able to program and run your power source remotely via PC. Easily gather data and analyze test results like never before.
Educational Resources
EEC understands that there is a lot to know when it comes to our Power Sources and medical device safety testing. Here are some educational materials that may help you with your testing.
Use of an Isolation Transformer While Performing Leakage Current or Functional Run Tests >